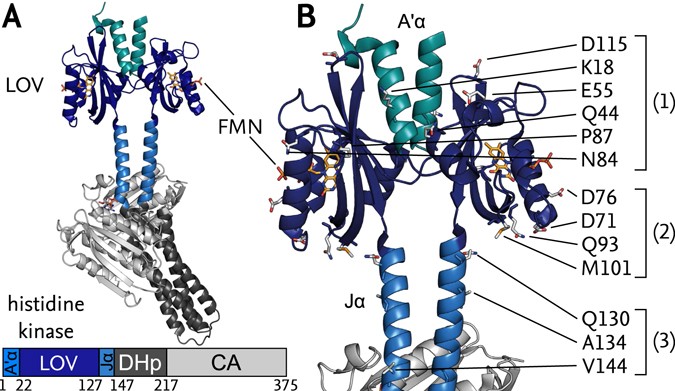
Structure of YF1. a Structure of YF1 in its dark-adapted state as

Download scientific diagram | Structure of YF1. a Structure of YF1 in its dark-adapted state as resolved by X-ray crystallography 13. The location of the different domains, of the flavin mononucleotide (FMN), of the cofactor adenosine diphosphate (ADP), and of the phosphoaccepting histidine 161 are indicated. b Light induced conformational changes of the LOV photosensor domain refined from X-ray solution scattering 22. The changes are maximal at the C-termini that feed into the Jα helices (dashed arrows). The coloring is according to the root mean square deviation of the alpha carbons from publication: Sequential conformational transitions and α-helical supercoiling regulate a sensor histidine kinase | Sensor histidine kinases are central to sensing in bacteria and in plants. They usually contain sensor, linker, and kinase modules and the structure of many of these components is known. However, it is unclear how the kinase module is structurally regulated. Here, we use | Secondary Protein Structure, Bacterial Proteins and Protein Conformation | ResearchGate, the professional network for scientists.
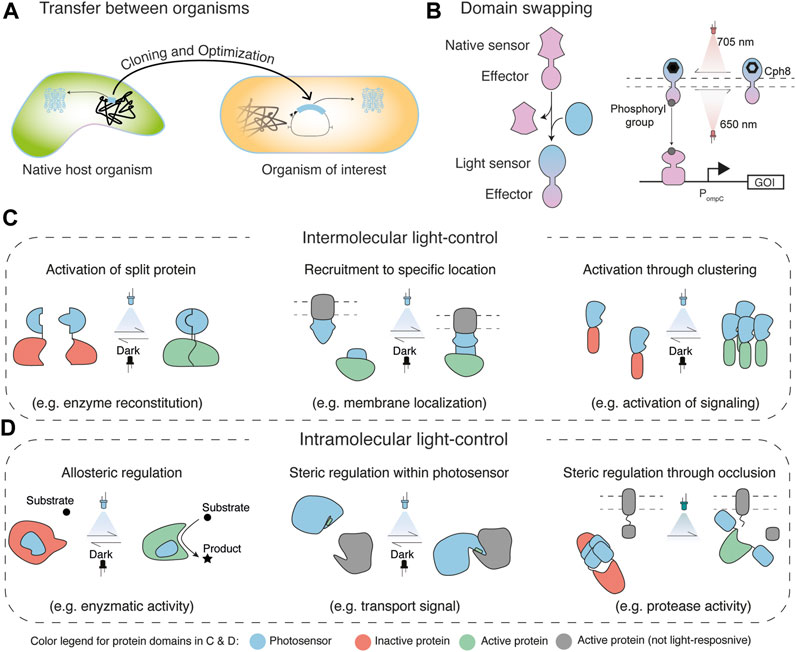
Frontiers Engineering Light-Control in Biology

Stephan NIEBLING, Postdoc, Applied Physics

Structure of the ATP synthase catalytic complex (F1) from Escherichia coli in an autoinhibited conformation

Staircase solutions and stability in vertically confined salt-finger convection, Journal of Fluid Mechanics

Full-Length Structure of a Sensor Histidine Kinase Pinpoints Coaxial Coiled Coils as Signal Transducers and Modulators - ScienceDirect
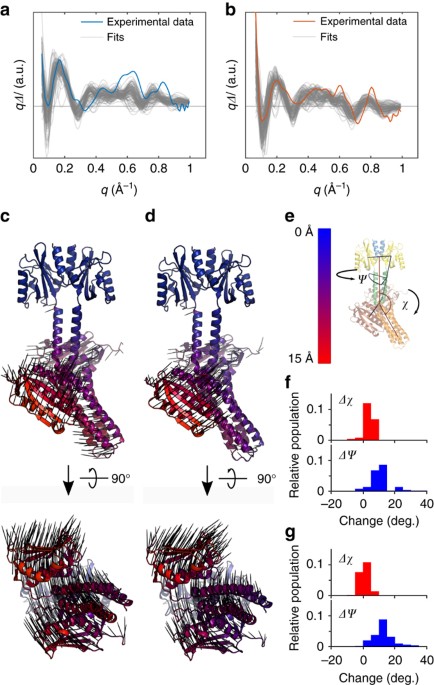
Sequential conformational transitions and α-helical supercoiling regulate a sensor histidine kinase

Gemma NEWBY, Application Scientist, PhD Chemistry, Xenocs, Sassenage, Science and Application

Stephan NIEBLING, Postdoc, Applied Physics
Blue-light reception through quaternary transitions







