What is the compressibility factor (Z) for 0.02 mole of a van der Waal

(d) (0.1+(1000xx(0.02)^(2))/(V^(2)))V=20xx0.02 =0.1V^(2)-0.4V+0.4=0 =V^(2)-4V+4=0 implies" "V=2L Z=(PV)/(nRT)=(0.1xx2)/(20xx0.02)=0.5

atm. 6. What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of lam. Assume the size of gas molecules is negligible. Given : RT = 20

Solved Data: Ideal gas constant R 8.314 J mol1 K1 1 (a)

Atomic Structure - Notes - LearnPick India

Poulduly 59. What is the compressibility fac is the compressibility factor (Z) 0.02 mole co Vanderwaals' gas pressure of 0.1 atm. Assume the size of gas molecules is negligible. . RT =

The van der Waals parameters for gases W, X, Y and Z are {:(Gas,a(

Filo Student Questions For CBSE , Grade 9
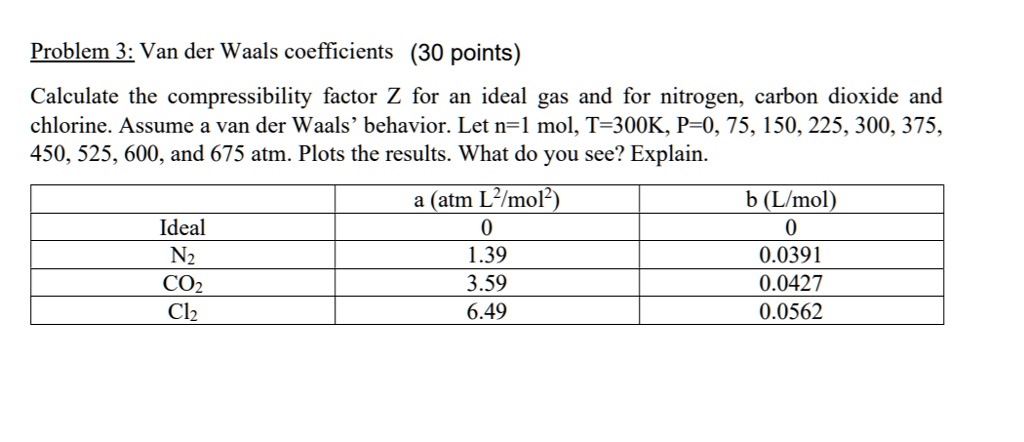
SOLVED: Problem 3: Van der Waals coefficients (30 points

atm. 6. What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of lam. Assume the size of gas molecules is negligible. Given : RT = 20

PEDULCE UUUUUUUU 3. A 1 litre vessel contains 2 moles of a vanderwaal's gas. Given data : a = 2.5 atm-Lmole - T= 240 K b = 0.4 L-mole- RT = 20

Atomic Structure - Notes - LearnPick India
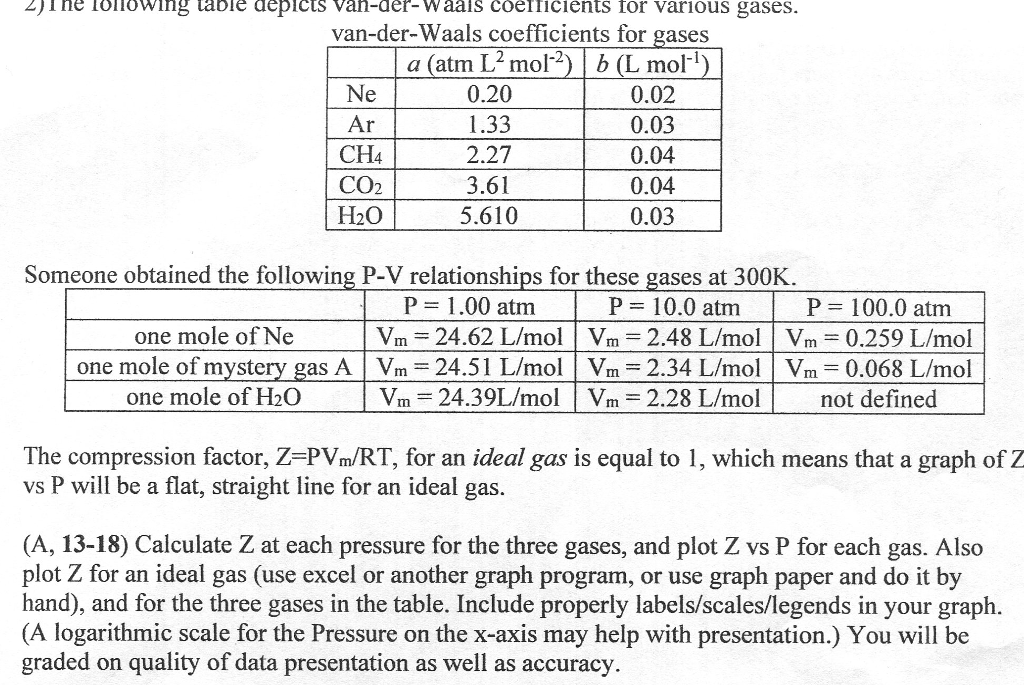
The following table depicts van-der-Waals coefficient
If Assertion is true statement but Reason is false, then mark (3)

The compressibility factor (Z) of one mole of a van der Waals' gas

PUUDU UUUUUUDUS ( U 13 SUMU U yuu puuuu What is the compressibility factor (Z) 0.02 mole of a van der Waals' gas pressure of 0.1 alm. Assume the size of gas






