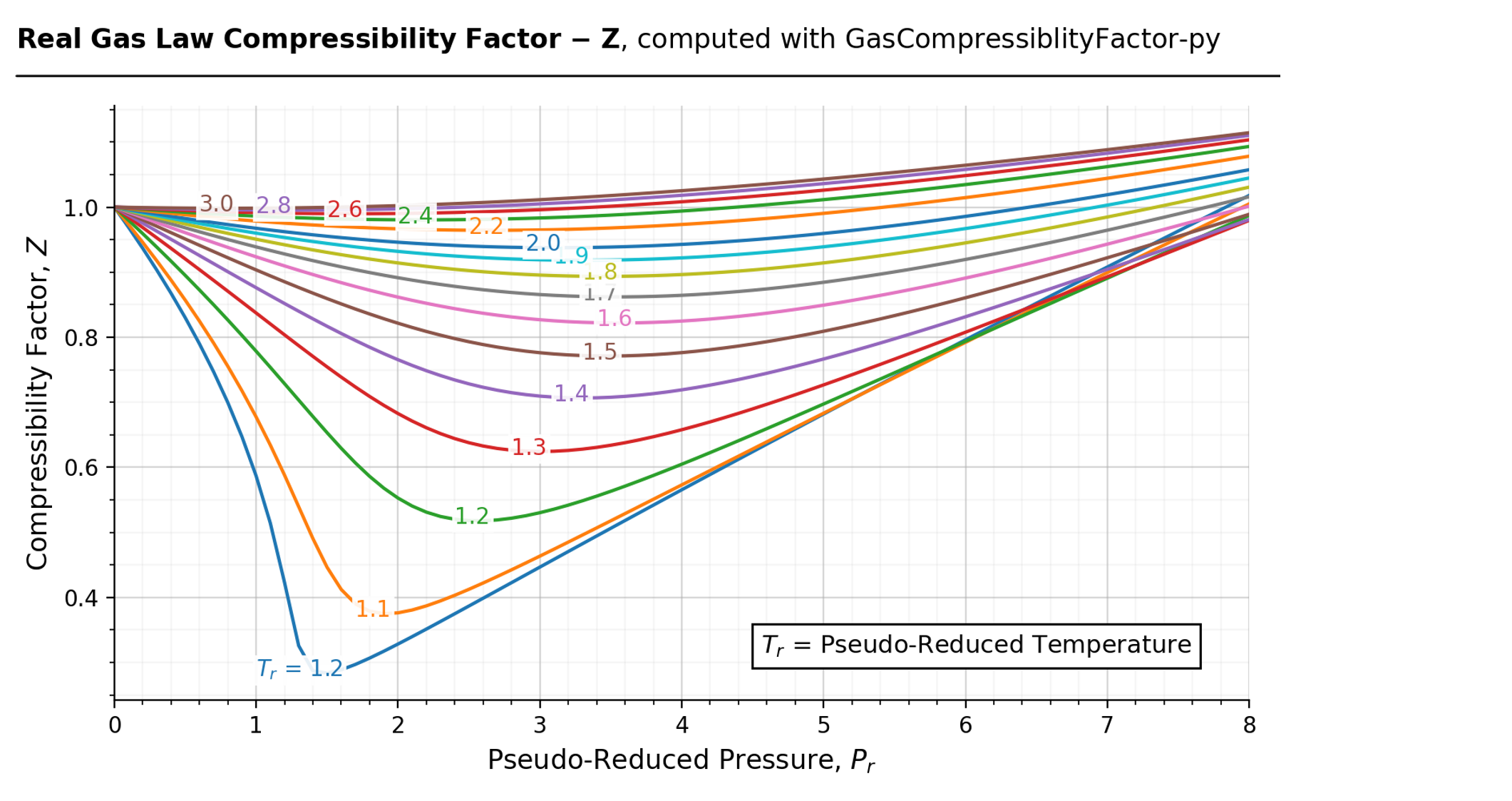
For a given gas, a graph is shown between compressibility factor

By the graph, at T2, the gas does not deviate from ideal gas behaviour at low pressure (Z = 1).Hence, T2 = TBT2 = TB (Boyle's temperature)T1 > TB (Here, Z > 1)This implies that T3 B and TB > TC(Here, Z So, T1 and T2 must be above TC.

e Compressibility factor (Z) for hydrogen WRT pressure and temperature

Compressibility factor for methane.
Punjabi] The graph of compressibility factor (Z) vs. P for one mole o
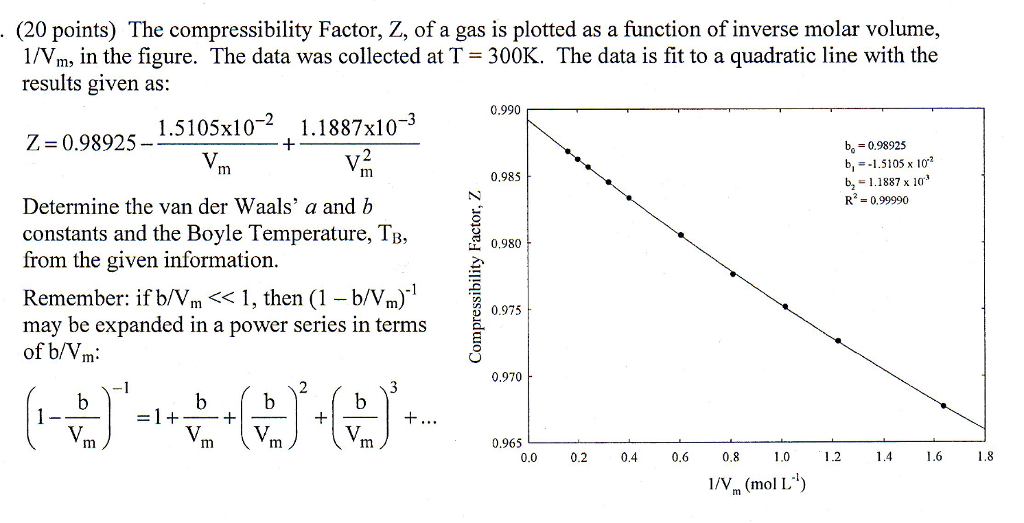
Solved The compressibility Factor, Z, of a gas is plotted as

1.3 Deviation from ideal gas behaviour

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Compressibility factor Z is plotted against pressure p for four different gases A,B, C D. The correct order of critical temperature of the gases shown in the above graph is

The given graph represents the variation of Z(compressibility factor =displaystyle frac{mathrm{P}mathrm{V}}{mathrm{n}mathrm{R}mathrm{T}}) versus mathrm{P}, three real gases mathrm{A}, mathrm{B} and C. Identify the only incorrect statement.For the gas C

Compressibility Factor Z Important Concepts and Tips for JEE Main