200 g of a sample of limestone liberates 66 g of CO2 on heating
200 g of a sample of limestone liberates 66 g of CO2 on heating. The percentage purity of CaCO3 in the limestone is Options:a 95
200 g of a sample of limestone liberates 66 g of CO2 on heating- The percentage purity of CaCO3 in the limestone is Options-a- 95

Topical Mock Chemistry Questions, PDF, Chemical Elements
Putting the Genie Back in the Toothpaste Tube

PDF) Quimica Analitica Hamilton
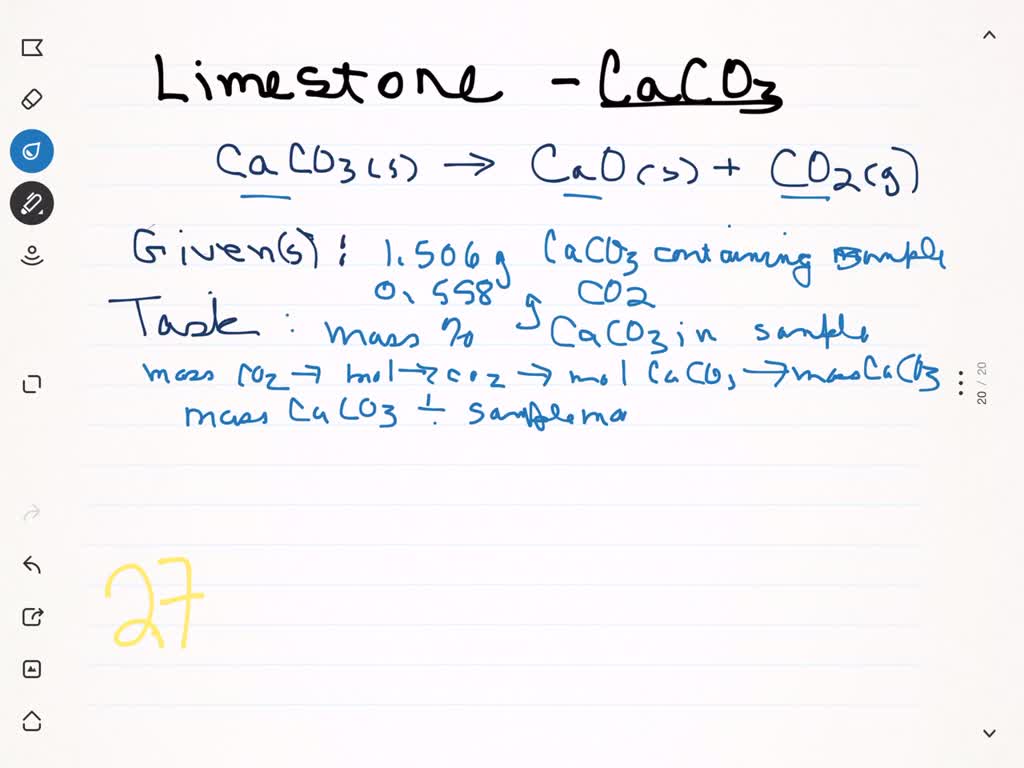
⏩SOLVED:A sample of limestone and other soil materials was heated,…

Calculate the enthalpy of the reaction 2NO(g) + O2(g) → 2NO2(g) g

Calculate the enthalpy of the reaction 2NO(g) + O2(g) → 2NO2(g) g

Alteration in molecular structure of alkali activated slag with various water to binder ratios under accelerated carbonation
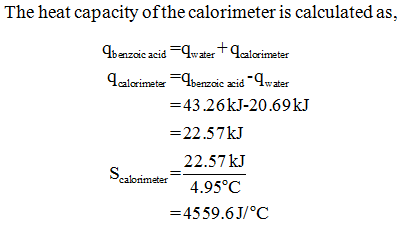
Answered: The heat of combustion of benzoic acid,…
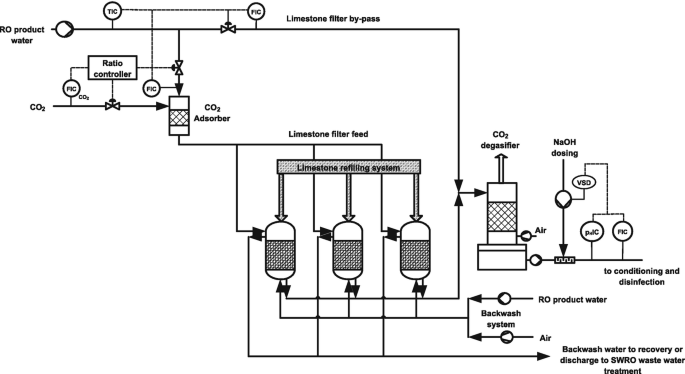
Post-Treatment

The role of hydrotalcite-like phase and monosulfate in slag cement paste during atmospheric and accelerated carbonation - ScienceDirect

Answered: The heat of combustion of benzoic acid,…
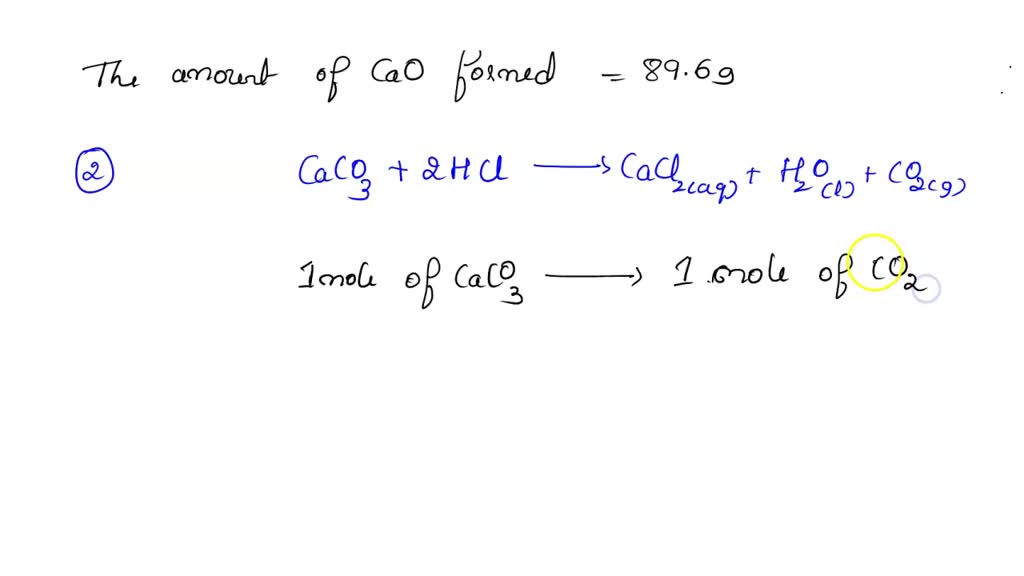
SOLVED: Calcium carbonate (limestone) decomposes when heated: CaCO3 CaO CO2 When 20.0g of calcium carbonate are decomposed, 11.2g of calcium oxide (lime), CaO, are formed. Calculate the mass of calcium oxide formed







